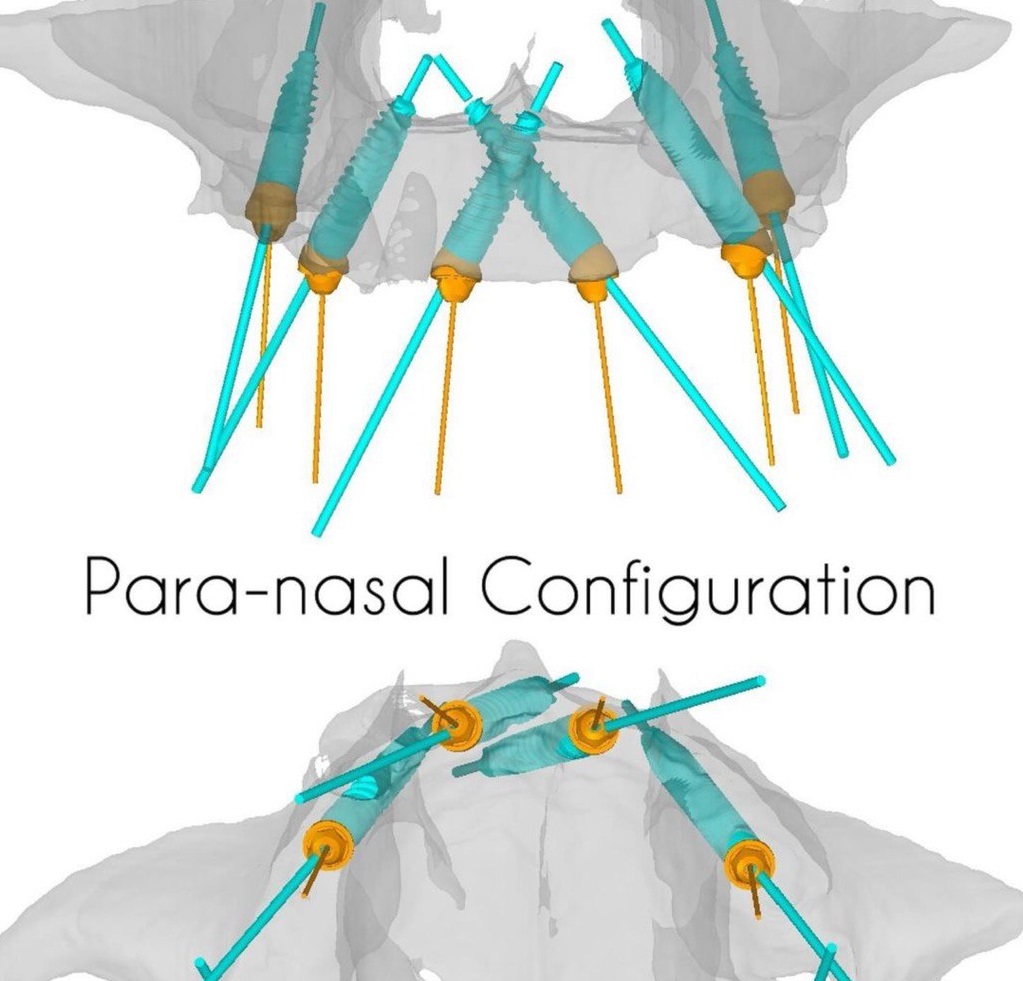
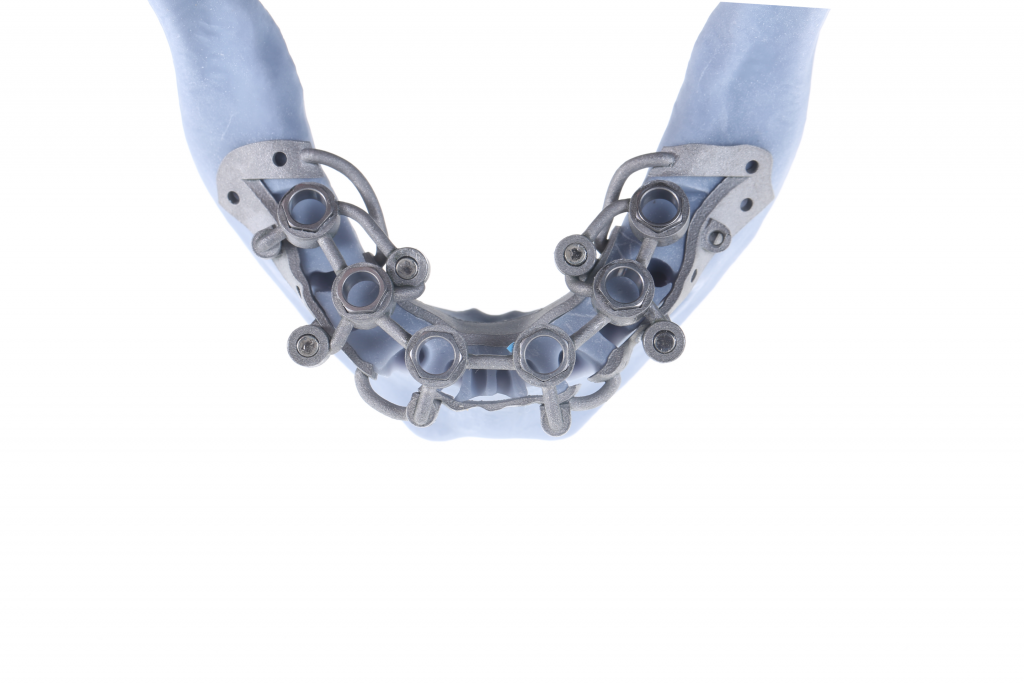
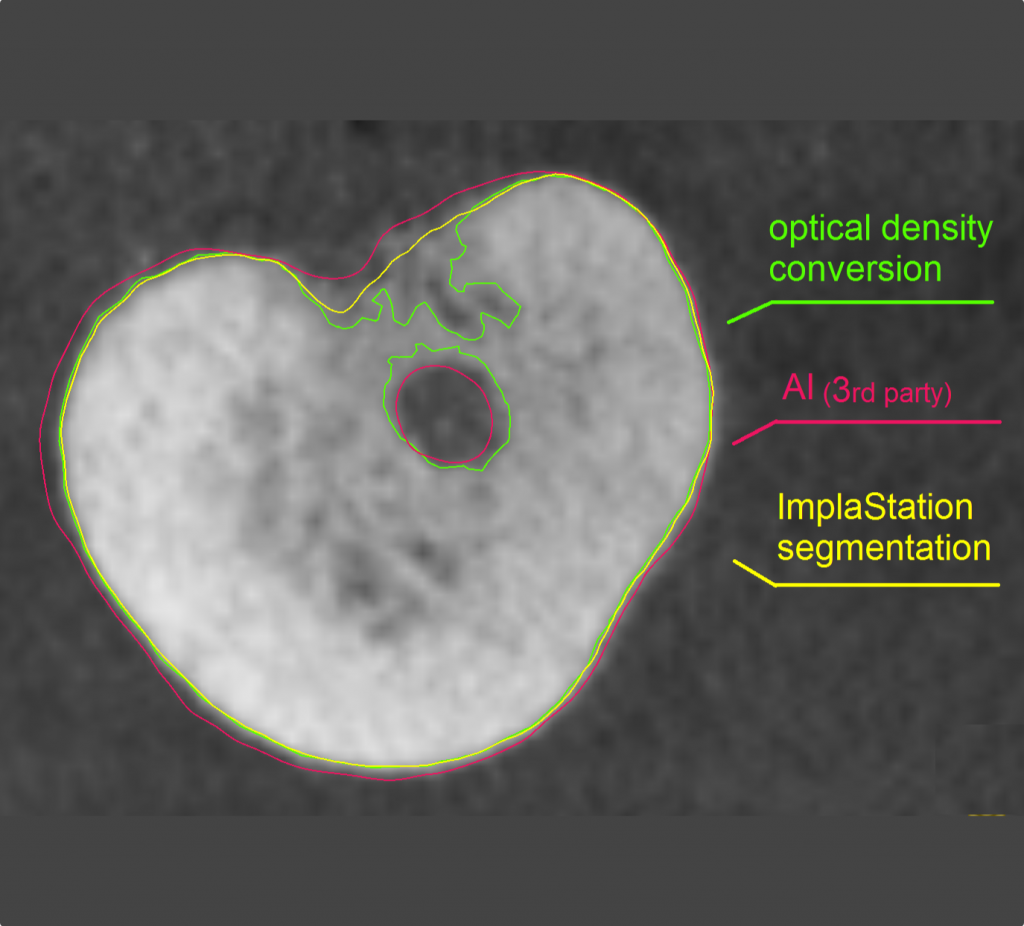
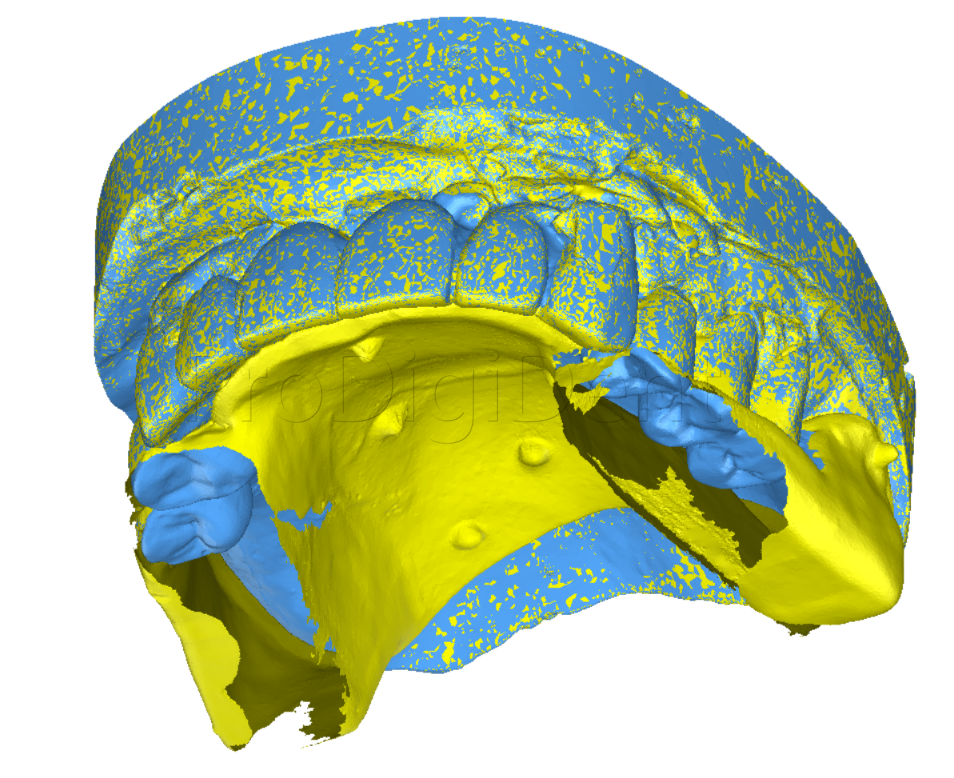
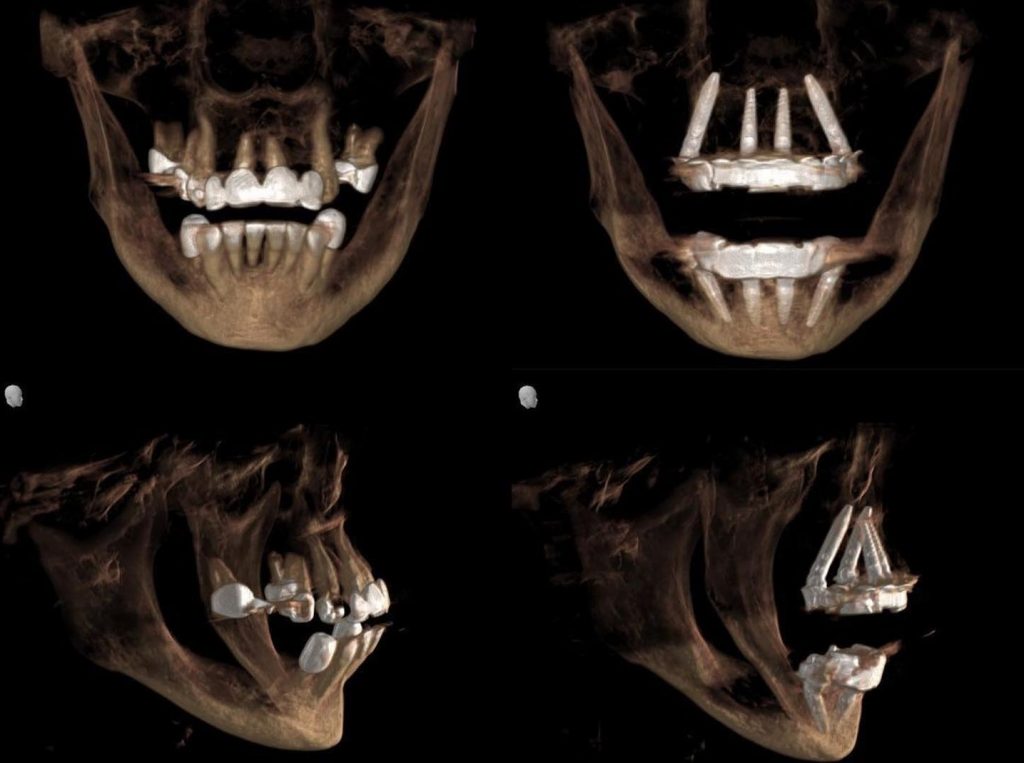
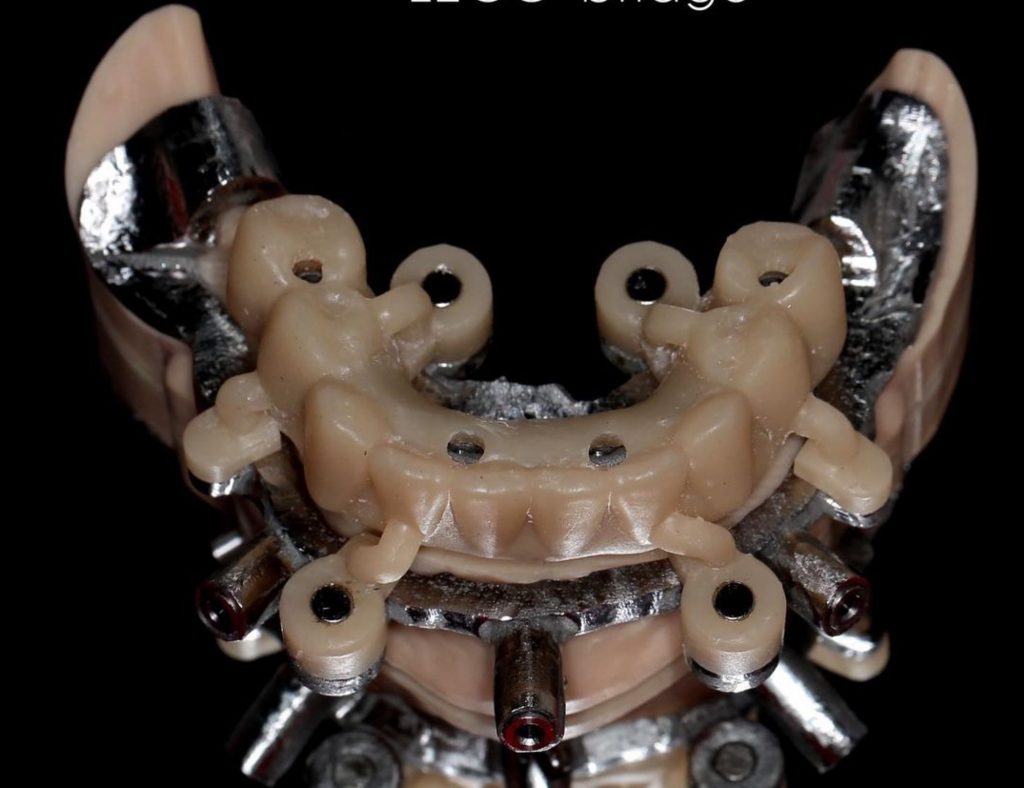
ImplaStation featuring implant planning and surgical guide design received FDA 510(k) market clearance!.
Prodigident is proud to announce that on December 3, 2021, the FDA (U.S. Food and Drug Administration) granted (510k) market clearance for the sale of the ImplaStation software in the United States.
TO ALL EVENTS